1 Introduction to the subject. Organism and its basic physiological functions
The organism is an open, self-existing, self-regulating unit of the organic world that reacts as a whole to changing environmental conditions. The organism can be studied at the systemic, organ, tissue, cellular and molecular levels.
The purpose of the lesson: to understand the purpose and objectives of physiology, to get acquainted with the physiological methods of research and the equipment for physiological experiments.
Basic equipment and general methodology for physiological studies
Physiological experiments are performed on live animals or on isolated organs. The choice of the animal is determined by the task of the experiment. In laboratory-practical studies, most experiments are performed on frogs. In some cases, experiments are performed on warm-blooded animals, as well as on humans (for example, physiology of the sensory organs, circulation, respiration). The fulfillment of many physiological experiments requires the restriction of mobility or complete immobilization: for this purpose, painkillers, narcotics and hypnotics are used. The most common methods of anesthesia in laboratory animals are as follows: inhalation, intravenous, intramuscular and subcutaneous. An example of inhalation anesthesia can be inhaling ether, chloroform or a mixture of the two by the animal, after being given narcosis the animal is fixed on the operating table. In chronic experiments (fistulas of the salivary, gastric, intestinal) limitation of mobility is achieved by the use of special restraining chairs.
Fixation of animals
Frogs. The frog is fixed on the cork board in the dorsal position, fixing the head and limbs with pins. If necessary, the frog is anesthetized by being placed under a small glass cap, where a piece of cotton wool soaked with ether or chloroform is placed, or, by the most common method, destroying the central nervous system by a probe.
Rabbits. The rabbit is fixed on a special table, fixing the head with a headholder with a ring. On each leg of the rabbit gauze collars attached to four metal braces are put. For rabbits urethane, hexenal and ether anesthesia are used.
- a) Urethane anesthesia: 10 % – hexenal solution is injected into the outer vein of the ear at a dose of 1.5 ml of solution per 1 kg of live weight;
- b) Hexenal anesthesia: 10 % – hexenal solution is injected into the same vein at a dose of 1 ml of solution per 1 kg of live weight;
- c) Ether anesthesia: cotton wool soaked with ether is brought to the nostrils of the rabbit. The cotton wool is periodically soaked with ether.
Cats. Because of the great aggressiveness, they are first anesthetized and then fixed.
- a) Ether-chloroform anesthesia: the cat is placed under a glass cap, where a piece of cotton wool soaked with ether chloroform is put (1: 1). After the cat falls asleep, it is removed from under the cap, fixed on the restraining chair and a mask is put on the face.
- b) Thiopental anesthesia: 2.5% – a solution of thiopental is injected into the thoracic cavity at a dose of 20 mg per 1 kg of live weight.
Dogs. In chronic experiments, they are fixed in restraining chairs in a standing position. The neb of the dog is strengthened with a tape loop, which is put on the front of the neb and fastened behind the back of the head. For vivisection, the dog is fixed in a special operating crate or in portable one (like a rabbit). For dogs, usually combined morphine-chloroform anesthesia is used, and thiopental anasthesia is used more rarely.
- a) Morphine-chloroform anesthesia: first, the animal is injected subcutaneously with 1% morphine solution in the amount of 1 ml per 1 kg of live weight. After 15-20 minutes, when the animal falls asleep, it is fixed in the restraining chair and given an inhalation anesthesia - a mixture of ether, chloroform and alcohol at a ratio of 3: 2: 1, respectively.
- b) Thiopental anesthesia: 2.5% – a solution of thiopental is injected into the thoracic cavity at a dose of 20 mg per 1 kg of live weight.
Birds. When carrying out experiments on birds with fistula (chickens, pigeons) a special restraining chair is used. It consists of a light metal frame, reinforced on 4 legs. A thick fabric with two holes for legs and a third hole for the fistula tube is stretched onto the frame. To limit the movement of the legs, they are tied to the legs of the chair (without strong tension). The movements of the wings are limited by a wide cloth tape stretched across the back. The ends of the tape are fixed on the frame of the chair. Experiments on birds are carried out, as a rule, without anesthesia.
The main instruments and equipment used in conducting laboratory studies
Various laboratory equipment and instruments are widely used in laboratory and practical studies in physiology.
This equipment can be conditionally divided into 3 groups: devices for stimulation by electric current; recording instruments; devices on which the record is kept.
An electric current is used to study the activity of organs and tissues in a physiological experiment. Most often in these experiments the electrostimulator is used.
Electrostimulator ISE-01. It has received the widest application in the educational process for determining the excitability of the nerve and muscle, obtaining single, tetanic contractions of muscles and other purposes.
Marey capsule (drum). With its help, functions are registered via air transmission. This method is used when there is some distance between the organ being examined and the recording instrument. By the method of air transmission it is possible to record the change in the volume of the organ (stomach, intestine, etc.) and blood pressure in the vessels. Using a Marey capsule and a rubber cuff filled with air, which Marey called a pneumograph, breathing can be recorded too.
Leverages are used in physiological experiment for recording movements of isolated organs (heart, intestine, uterus, etc.). An example is the Engelman two-arm lever.
Myograph allows to record the contractions of an isolated muscle.
Time indicators. To judge the time of the process registered on the kymograph, and to mark the beginning and the end of the irritation, time indicators are used. The electromagnetic indicator serves to mark the irritation. It is also used to mark time. In this case, the indicator is connected to an automatic interrupter.
Tripods are used for fixing the registered devices. The most convenient is a universal tripod.
Instruments on which the recording is conducted. The oscillations of the levers fixed on the tripod, with the help of a scribe, are recorded on a kymograph on white paper using a special scribe.
Kymograph consists of a removable rotating drum, which is put on the axis of the tripod. The drum is strengthened from above and below by screws that allow it to be moved to a vertical position. From the bottom, a metal disk is mounted on the axis, rotating as a result of friction of the roller along its lower surface. The roller is driven by a clock mechanism mounted on a tripod in a protective casing. The rotation speed of the kymograph drum can be adjusted by changing the distance from the axis to the movable roller by moving it. In addition to the described instruments and devices on laboratory and practical classes, tools for preparation are needed.
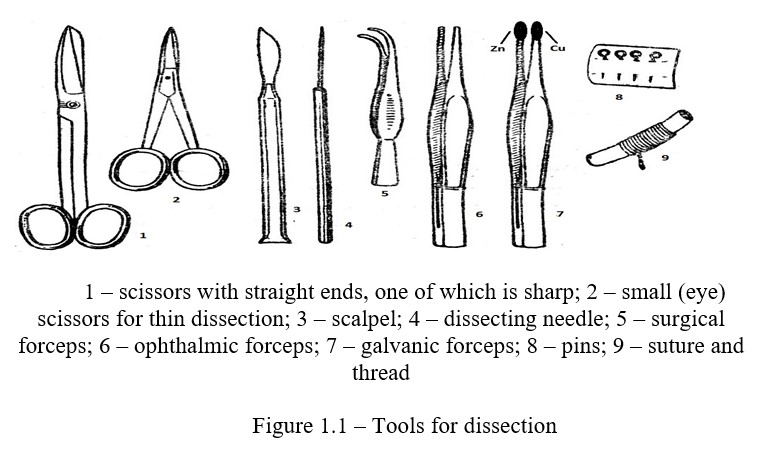
Cannulas and catheters. In many physiological experiments on animals for direct measurement and recording of arterial and venous pressure, recording respiratory movements, obtaining large quantities of blood from blood vessels, collecting secretions of digestive glands, etc. appropriately made tubes, called cannulas are used. At present, many methods of experimental physiology are modified, including angiostomy (cannulation of vessels), a new variant is born - acute and chronic catheterization of blood vessels, including lymphatic vessels, which greatly facilitates the collection of blood (lymph) samples from deep vessels in a sufficiently large volume and at frequent intervals. At the same time, it is possible to simultaneously catheterize arterial, venous and lymphatic vessels, which is very valuable in the study of intermediate metabolism. The length of the catheter depends on the purpose of the operation and the size of the animal.
Physiological solutions.
Saline solutions are very widely used in physiological experiments. They are used as blood replacement solutions in acute and chronic experiments, used for perfusion of isolated organs, moistening the surface of drying tissues during surgery and experiments. In experiments and clinics, isotonic solutions are often used (the osmotic pressure of which is equal to the osmotic pressure of the blood). These solutions are prepared according to the instructions of Ringer, Locke and Tyrode (Table 1.1).
Table 1.1 – Composition of solutions for physiological studies, %
|
Solutions |
NaCl |
KCl |
CaCl2 |
NaHCO3 |
Glucose |
MgCl2 |
NaH2PO4 |
|
For frog tissue |
|||||||
|
Physiological |
0,65 |
- |
- |
- |
- |
- |
- |
|
Ringer |
0,6 |
0,0075 |
0,01 |
0,01 |
- |
- |
- |
|
For mammalian tissue |
|||||||
|
Physiological |
0,85 - 0,9 |
- |
- |
- |
- |
- |
- |
|
Ringer |
0,9 |
0,042 |
0,024 |
0,02 |
- |
- |
- |
|
Locke |
0,9 |
0,042 |
0,024 |
0,015 |
0,1 |
- |
- |
|
Tyrode |
0,8 |
0,02 |
0,02 |
0,1 |
0,1 |
0,01 |
0,005 |
Control questions
- The subject and tasks of physiology?
- The main stages of the development of physiology?
- The role of I.M. Sechenov and I.P. Pavlov in the development of physiology. Famous physiologists of Kazakhstan?
- Methods of physiological studies?
- Homeostasis and its indices?
- Basic concepts of physiology: function, physiological system, functional system?
- The main links of the functional system (according to P.K. Anokhin)?