2 Physiology of excitable tissues
2.1 Preparation of a neuromuscular preparation. Properties of excitable tissues
To study the basic physiological properties of living tissues, frog tissues are most commonly used. The wide use of the nerve-muscle tissue of the frog is due to the fact that they, being isolated from the body, retain their vital properties much longer than the tissues of warm-blooded animals. Nervous and muscular tissue in the body, are functionally interconnected, they respond to the action of stimuli by a definite reaction, providing the presentation of various types of movement. As a result of the stimulation, a process of excitation appears in the tissues, which is accompanied by a specific function peculiar to each tissue. Thus, when the muscle tissue are irritated, the fibers contract, and when the neural tissue are stimulated, impulses emerge, and they are transmitted to the appropriate organs. A convenient model to study these processes is a neuromuscular preparation consisting of the gastrocnemius muscle, femoral bone, sciatic nerve together with a piece of spine. The excitability of the tissue is usually measured by the threshold of force and the threshold of the operating time of the stimulus necessary to bring the tissue into a state of excitation. The threshold of force is that minimal stimulus force, which causes a minimal contraction effect. The time threshold or useful time is the minimum time during which the stimulus of the threshold force should act to induce excitation.
The purpose of the lesson: master the technique of preparation of the neuromuscular preparation, get acquainted with the reaction of the tissue to various types of stimuli and their different force, learn to evaluate the excitability of the neural and muscle tissues by determining excitability during direct and indirect stimulation of the muscle, and make sure that the magnitude of the contraction depends on the strength of the stimulus.
The following is necessary for work: frog, surgical set (scissors, forceps, scalpel) tripod, electrostimulator, kymograph, physiological solution, crystals of table salt.
Work 1. Preparation of the neuromuscular system
All works on the physiology of excitation are performed on a neuromuscular preparation consisting of the gastrocnemius muscle, femoral bone, sciatic nerve, along with a piece of the spine.
Work progress: Take the frog, wrap it in a gauze cloth so that the forelegs of the frog are pressed to the body, and the head remains free (Figure 2.1). Immobilize the frog by destroying the brain and spinal cord.
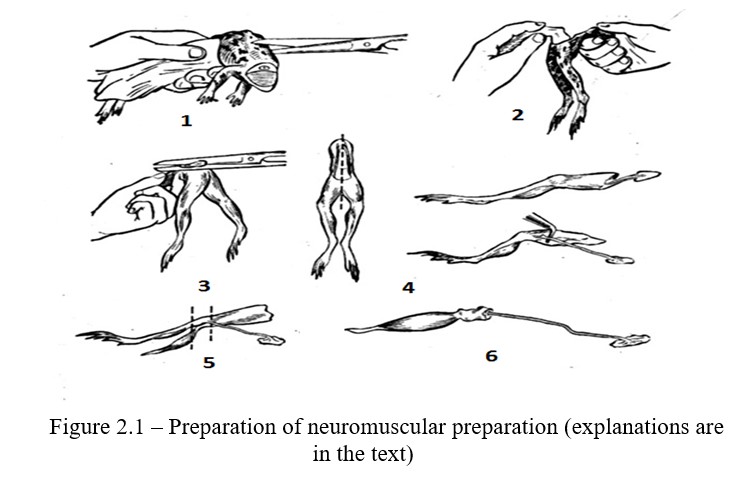
After the destruction of the spinal cord, remove the needle from the spinal canal and check for reflexes. Take the frog by its hind legs so that its abdomen hangs, cut the vertebral column across; make two incisions on the right and left of the spine, cutting the skin together with the muscle until the symphysis (Figure 2.1, 1). Cut and remove the hanging insides, as well as the skin and muscles of the abdominal wall so that the hind limbs with the sacrum, a piece of spine and the sciatic nerves leading out from preserved remains of the spinal canal remain. Hold the spine with one hand and with the other one grab the edge of the skin with a cloth and remove it. Obtained is the preparation of the two hind legs of the frog (Figure 2.1, 2).
Preparation of the rheoscopic leg. The preparation of the hind legs of the frog should be taken behind the spine and bend so that the coccyx protrudes upward (Figure 2.1, 3). Cut the coccyx out using scissors. Place the preparation with the ventral side up. Try not to touch the nerve stems of the sacral plexus, cut along the middle line of the spine and separate the legs from each other. While holding the rest of the spine, separate the sciatic nerve using glass hooks. Remove the pelvic bone by cutting it near the spine and hip joint (Figure 2.1, 4).
Preparation of a neuromuscular preparation. The next stage of the work is the dissection of the sciatic nerve and gastrocnemius muscle. For the dissecion of the nerve, place the thigh its posterior surface is upward, pull the muscles apart and dissect the underlying sciatic nerve along its entire length. Raise the nerve holding the rest of the spine, carefully cut surrounding tissue with scissors. Cut the leg one and a half centimeters above the knee joint (Figure 2.1, 5). Remove the rest of the femoris muscle. Draw a scissor blade under the Achilles tendon, separate it along the entire length, and cut it below the sesamoid bone. By grasping the tendon with forceps, pull the gastrocnemius muscle to the side, tearing the fascia that connects it to other tissues. Cut the leg below the knee joint. The neuromuscular preparation of the gastrocnemius muscle and sciatic nerve is obtained (Figure 2.1, 6). The preparation should be placed in a Petri dish containing Ringer's solution or 0.6% solution of sodium chloride.
Work 2. Determination of the threshold of muscle excitability for direct and indirect stimulation
Excitability can be judged by the magnitude of the threshold of excitability. The contraction of the muscle can occur when the current is directly applied to it – direct irritation, or irritation on the nerve that goes to the muscle – indirect irritation.
Work progress: For work, use a neuromuscular preparation. Prepared neuromuscular preparation is strengthened by the femur in the clamp. Puncture Achilles tendon with a hook and attach it to the recording lever. Lay the nerve on the substrate. Carefully, not violating the integrity of the preparation, bring the thymic electrodes connected to the terminals of the electrostimulator to the nerve (Figure 2.2).
The electrodes of the electrostimulator are applied directly to the muscle of the neuromuscular preparation and find the magnitude of the threshold for the muscle in the same way as for the nerve.
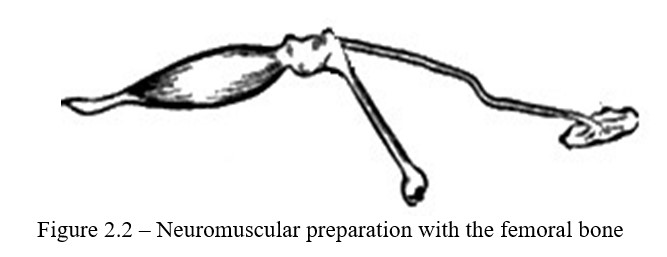
Determination of the excitability threshold should begin with a minimum voltage, therefore, set the range switch to position 0.01 and turn the "amplitude" handle to the right until the muscle begins to contract. If the muscle does not contract even with the rightmost position of the handle, return it to position 0, set the range switch to 0.1, then increase the amplitude to a value at which the muscle contracts for the first time. If there is no effect, put the range switch to position 1 and repeat the determination. Thus, a minimum strain, in which excitation appears in the nerve will be found. Then transfer the electrodes to the muscle and in the same way find the threshold of excitability with direct muscle stimulation.
Work 3. The study of the effect of various stimuli on the nerve and muscle
All stimuli can cause the excitation of living tissue, the muscle responds to the mechanically, electrical, chemical stimuli by contraction; nervous tissue responds by impulse of excitation.
Work progress: A single stimulation of the current is applied to the sciatic nerve of the neuromuscular preparation, which leads to an instant contraction of the muscle. The nerve is squeezed with forceps and the muscle responds to this irritation by contraction. Crystals of table salt are put on the nerve. After some time the muscle will begin to rapidly contract, the contraction will continue after the removal of the crystals of salt.
Work 4. Determination of the dependence of the magnitude of the contraction on the strength of stimulation
Work progress: The threshold strength of the stimulus is found by means of an electrostimulator and the curve of muscle contraction is recorded. As the current increases, the height of the muscle contractions increases - superthreshold reduction . The current strength is increased until they receive 2-3 contractions of the same height. The force of maximum contraction (optimum force) is marked. With a further increase in the intensity of the current, the magnitude of the contractions will gradually decrease (the strength of the pessimal effect is noted).
Control questions
- The concept of excitable tissues, their properties?
- Tissue excitability and measurement methods?
- Change in excitability upon excitation?
- Criteria for assessing excitability?
- The main laws of irritation (the law of force, force - time)?
2.2 Bioelectric phenomena in living tissue
Excitation in the tissue is accompanied by the appearance of an electric current, which is called the "current of action". The main physiological significance of "action currents" is their participation in conducting excitation in tissues. The appearance of action currents is understood as the appearance of a potential difference between an excited tissue site and a resting site. In the tissues of the organs, electrical phenomena can arise, the nature of which is not related to their excitation - these are the so-called "resting currents." These currents arise as a result of the potential difference between the outer and inner surface of the cells of the organs tissues.
The purpose of the lesson: Make sure with a muscle in the presence of biological currents in living tissue and show the occurrence of the action currents and rest currents.
The following is necessary for work: frogs, a set of tools, electrostimulator, balcony, galvanometer, glass hooks, slide glasses, Ringer's solution.
Work 1. The first Galvani experiment on the basis of which the question about the presence of animal electricity was raised
Work progress: Take the frog and destroy the brain, cut the back across the vertebral column by 0.5-1 cm above the pelvic-thoracic articulation. Grab the rest of the spine with a cloth, remove the skin from the hind limbs using forceps, and remove the remains of the intestines. Lead an aluminum hook of the balcony under the sciatic nerve and raise it. The movement of the hand brings into contact the frog's legs with copper plastic. This creates a current that irritates the neuromuscular preparation and the muscles contract. The appearance of this current explains the potential difference of 2 different metals.
Work 2. The second Galvani experiment (experiment without using metals)
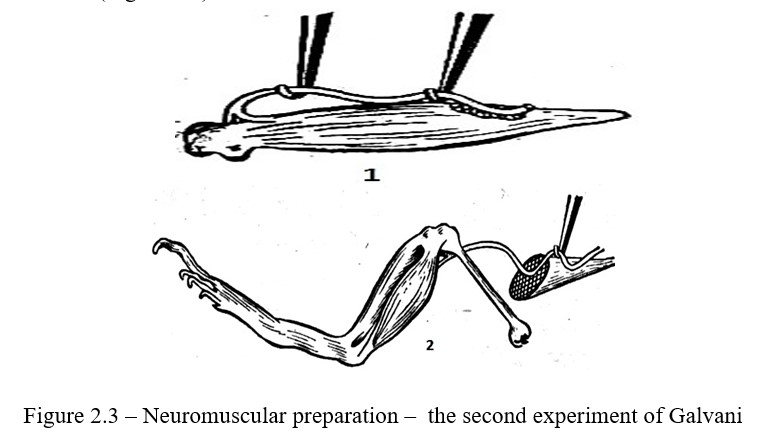
Work progress: The sciatic nerve is dissected to the knee joint using a glass hook, while fully retaining the leg. Cut the muscle around the Achilles tendon with scissors; lay the preparation on a slide plate. Lift the nerve with one hook, and use the second hook to throw the nerve onto the muscle so that part of the nerve gets to the damaged area, and another part to the undamaged muscle segment. At the moment of nerve throwing, the dactyls on the leg contract (Figure 2.3).
Work 3. Detection of static current with a galvanometer. The first experiment of Matteucci
Work progress: Prepare a neuromuscular preparation, damage the muscle around the Achilles tendon. Connect non-polarizable electrodes to the damaged and undamaged parts of the muscle, connecting to a needle galvanometer. The galvanometer needle deflects to the fault side, showing the static current (injury current).
Work 4. The second experiment of Matteucci (experiment with the secondary tetanus), allowing to detect the action current
Work progress: Prepare two neuromuscular preparations; put them on dry slide glasses so that they do not touch each other. The nerve of the second neuromuscular preparation is applied to the gastrocnemius muscle of the first neuromuscular preparation, and the nerve of the first neuromuscular preparation is irritated by induction current. In response to nerve irritation, the muscles of both preparations contract (Figure 2.4).
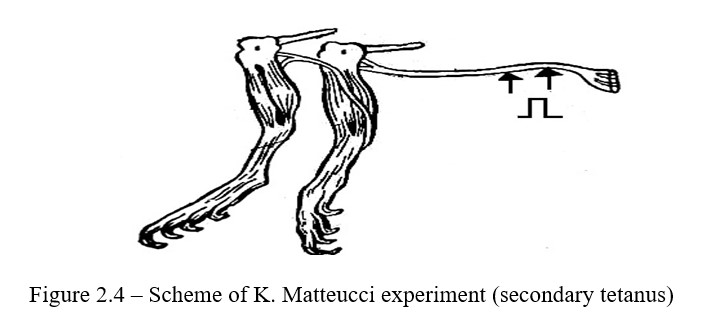
The irritant of the second nerve is the action current that occurs when the first neuromuscular preparation is excited. The excited part of the tissue is electronegatively charged, and the unexcited one, i.e. resting area is electropositively charged (Figure 2.4).
Work 5. Du Bois-Reymond experiment
Work progress: The experiment is carried out on the same neuromuscular preparation. The nerve is placed on the electrodes of the induction coil and irritated. Electrodes of the galvanometer are applied to the surface of the muscle. The needle of the galvanometer alternately deflectes to both sides from zero, showing the presence of a two-phase current.
Control questions
- Resting potential. Methods of registration. Ion-membrane theory of origin?
- Ionic excitation mechanism. The potential of action and its components. Change in excitability upon excitation?
- Theories explaining the nature of the origin of biocurrents?
2.3 Resistance to fatigue of the nerve and muscle fatigue. The doctrine of parabiosis
Nerve fibers, forming the peripheral nerves and conducting paths of the CNS, provide a fast transmission of information about the state of various structures of the body. Studying the laws of conducting a nerve impulse makes it possible to obtain an important material for understanding the basics of motor activity.
The doctrine of parabiosis. Fatigue is a temporary decrease in the working capacity of the cell of the organ or of the whole organism that comes with prolonged work. Fatigue develops with unequal speed in various excitable systems. In the "nerve-minoneural synapse-muscle" system, the myoneural synapse is fatigued first as a link with the lowest lability. In this case, excitability decreases, and finally there comes a complete loss of excitability, leading to the termination of the function. N.E. Vvedensky, by convincing experiment, proved the practicalresistance to fatigue of the nerve.
N.E. Vvedensky, 1901-1902, by investigating the effect of prolonged stimulation on the nerve by current or chemical substances, has shown that lability, excitability and conductivity sharply decrease in the altered area of neuron. Vvedensky called this state as parabiosis, which means the state between life and death. Parabiosis, according to Vvedensky, is a special stagnant form of excitement, which turns into inhibition. Thus, by the doctrine of parabiosis he discovered the nature of inhibition and that the transition of excitation to inhibition occurs through three phases: equalizing, paradoxical, inhibitory. Parabiosis can be caused by various factors.
The purpose of the lesson: establish the gradual development of parabiosis. Realize the resistance of the nerve to fatigue and muscle fatigue.
The following is necessary for work: frogs, preparatory set, set of instruments, tripod, myograph, electrostimulator, kymograph, Ringer's solution, cotton wool, ether, 2 gr weight.
Work 1. Obtaining parabiosis
Work progress: Prepare a neuro-muscular preparation with a long nerve and fix it in a myograph, place the nerve on the electrodes. Determine the excitation threshold during indirect stimulation. Record tetanic muscle contractions on the kymograph, irritating the nerve with a rhythmic induction current of medium and maximum strength. Notice the dependence of the height of the tetanus on the strength of the stimulus applied to the nerve. Then put a cotton wool soaked with ether or 0.8% solution of potassium chloride under the nerve between the irritating electrodes and the muscle, and observe the parabiosis that develops in the nerve. The process can develop slowly, so the first stage can be identified after 5-10 minutes. To do this, irritate the nerve above the injury site using induction current and record the muscle contractions to the threshold, average and maximum current strength. Now the excitement from the irritated site of the nerve must pass through the altered area before reaching the muscle.
On the kymograph will be recorded the following:
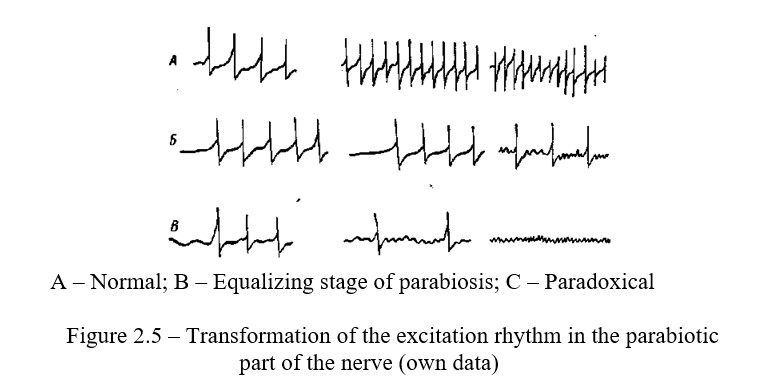
1) An equalizing phase of parabiosis when the muscle gives the same height of the tetanus at different strengths of the current applied to the undamaged area of the nerve (Figure 2.5, A);
2) Paradoxical phase of parabiosis that develops a few minutes after the equalizing phase and is characterized by the fact that high current strength gives a contraction smaller in strength (Figure 2.5, B);
3) The inhibitory phase of parabiosis when the impulses in the altered nerve region cease and the muscle does not respond to stimuli (Figure 2.5, C).
After the onset of complete parabiosis, remove the cotton wool and wash the nerve with physiological saline and again irritate the nerve with a current of different strength, observe the reverse development of the stages of parabiosis and restoration of the normal excitability of the nerve, thus making sure that the parabiosis process is reversible.
Work 2 Vvedensky's experiment showing a relatively low fatigue of the nerve
Work progress: Prepare a neuromuscular preparation, fix it in a myograph. Place the nerve of the preparation on the far electrodes. On the site of the nerve, closer to the muscle, put a piece of ice to create a block. Make sure the block is working, turn on the current and apply irritation to the nerve for 10 minutes. After 10 minutes, remove the block. Apply single irritations to the nerve and the muscle will respond with a contraction - this indicates that the nerve is practically untiring.
Work 3 Monitoring the localization and development of fatigue in the neuromuscular preparation
Work progress: A neuromuscular preparation is prepared and fixed in a myograph. A weight (2 g) is suspended from the muscle and a muscle contraction is recorded on the kymograph during intermittent stimulation. The curve of muscle contraction is recorded before the onset of muscle fatigue. When the muscle stops contracting, another pair of electrodes is applied directly to the muscle. Irritation is applied, to which the muscle responds by contraction. This indicates that fatigue is localized not in the muscle, but in the terminal neural plastic.
Control questions
- Classification of nerve fibers, peculiarities of conduction of excitation on medullated and nonmedullated nerve fibers?
- What are the physiological properties of nerve fibers?
- The laws and mechanism of excitation along the nerve fiber, afferent and afferent conductors?
- What is the rate of excitation on various nerves?
- Mediator that provides transmission of excitation from the nerve to the muscle?
- Vvedensky's parabiosis, its stages, significance for the theory and practice of medicine. System organization of conductive paths?
2.4 Physiology of the motor apparatus
Skeletal muscles consist of a large number of individual muscle fibers, diameter of which varies from 10 to 100 microns. Unlike the heart and smooth muscles, they do not have the automaticity, and their activity arises under the influence of impulses coming from the central nervous system. The mechanism of excitation and conduct of the latter is due to the processes occurring in the surface membrane of the fiber, and the contraction is carried out by the myofibrillar apparatus. The conjugation between them is achieved due to the functioning of the intracellular structure - the sarcoplasmic reticulum (SPR).
The main property of muscle tissue is the contraction occurring in response to irritation. There are several types of contractions: isotonic, isometric, single and tetanic contractions. Single contraction only occurs in experiments. This is due to the fact that under the conditions of the whole organism a single stimulation from the central nervous system to the muscle is accompanied by a volley of impulses.
The purpose of the lesson: To get acquainted with the types and methods of recording muscle contractions and to investigate the mechanism of appearance of the tetanus and the summation curve.
The following is necessary for work: frog, set of tools, tripod, myograph, electrostimulator, kymograph, Ringer's solution.
Work 1 Recording a single muscle contraction and curve analysis
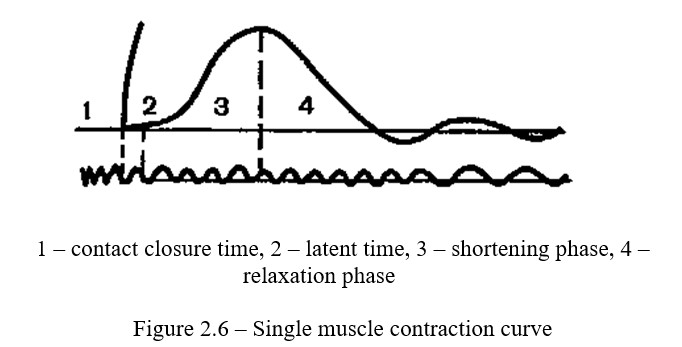
Work progress: A neuromuscular preparation is prepared and fixed in a myograph; the nerve is placed on electrodes of a single stimulus. An additional contact fixed to the base of the kymograph, is successively introduced to the primary circuit. The kymograph is placed in such a position that this contact is closed. By closing and opening the key in the primary circuit, strength of the stimulation is selected, at which the opening inductive impacts cause the maximum contraction, and the closing impacts remain below the threshold. On a curve giving 100 oscillations per second, time is recorded with a tuning fork. It is possible to record the time with an electric meter connected through a step-down transformer to a city network. Motor for 2-4 myograph and timer recorders are led to the surface of the kymograph and the single-contraction curve is recorded. In this case, the additional contact is broken, the drum of the kymograph is led to a rapid and smooth movement, as a result of which the circuit closes and a single irritation is applied to the muscle. After recording, the curve contraction should be analyzed and recorded (Figure 2.6).
Work 2 The sum of muscle contractions
The sum is the imposition of one single contracion onto another.
Work progress: The induction coil is connected to the upper terminals; the threshold strength of the stimulation is set. The interval between the stimuli used to get incomplete summation - more than 0.5 seconds, and for the total summation - less than 0.05 seconds. The contractions of the muscles will overlap one another and increase in height, because each subsequent impulse falls into a phase of increased excitability (the phase of exaltation).
Work 3 Obtaining the curve of a serrated and smooth tetanus
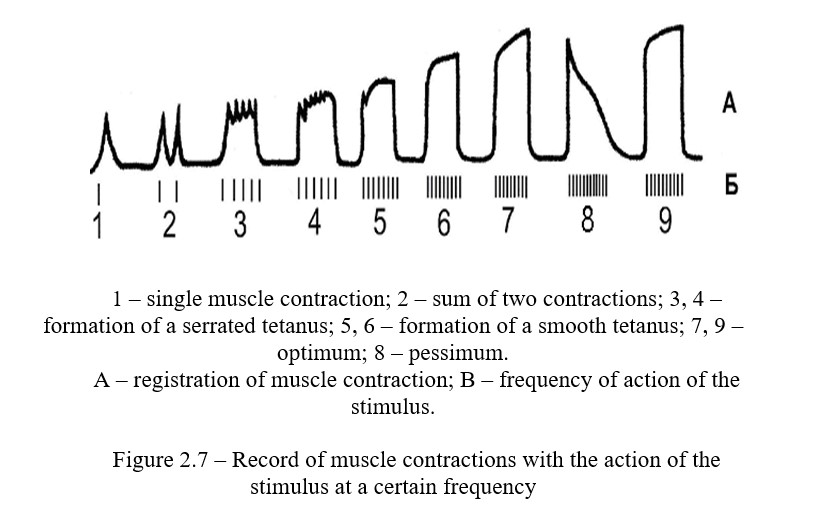
Tetanus is a prolonged muscle contraction, which is the result of summation of single contractions. There are serate tetanus and solid one (Figure 2.7).
Work progress: A neuromuscular preparation which is fixed in the myograph is used for work. The nerve of the preparation is put on the electrodes from the stimulator. The frequency of stimulation is set at 1 Hz, pulse duration is 1 ms, and the threshold of excitability (smooth rotation of the amplitude handle) on single pulses is found. Make the current strength a little bit more threshold, use the kymograph drum and record single muscle contractions. Then increase the frequency of irritation: switch the "frequency" handle by one division each time when recording. Before increasing the frequency, the stimulant must be switched off every time. Record a serrated and smooth tetanus. Find the optimal frequency of stimulation, to which the muscle responds with a smooth tetanus of maximum height - the optimum (approximately 40-50 Hz). Record the optimum for 4-5 seconds, and then sharply increase the frequency of stimuli to 100 and more Hz and record the frequency pessimum. After 5 seconds, reduce the frequency of stimulation to the optimal level and re-record the tetanus (it should be optimal) (Figure 2.7).
Work 4 Work of muscles under different loads
Since the main task of skeletal musculature is the performance of muscular work, in experimental and clinical physiology the amount of work done by the muscle and the power it develops during work are estimated.
According to the laws of physics, work is the energy spent on moving a body with a certain force over a certain distance: A = FS. If the contraction of the muscle takes place without load (in isotonic mode), then the mechanical work is zero. If the muscle is not contracted under the maximum load (isometric mode), then the work is also zero. In this case, the chemical energy is completely converted to thermal energy. According to the law of average loads, the muscle can perform maximum work under loads of medium magnitude.
The purpose of the lesson: Determine the dependence of the amount of muscle work on the magnitude of the load. Pay attention to the rule of average loads.
The following is necessary for work: frog, gauze pads, preparation tools, plate for dissection, Petri dish, electrostimulator, electric wires, kymograph, vertical myograph, pen, ink, 0.6% sodium chloride solution.
Work progress: When contracting and lifting a load, the muscle performs a certain work, which can be calculated, knowing the weight of the load and the amplitude of muscle contraction by the formula: A = P x h. The work is expressed in kg / m or g / cm; where P is the weight of the load, h is the magnitude of the muscle contraction. H = (H x s) / S, where H is the hight of the recorder in cm on the record on the kymograph drum; S is the length of
the recorder from the point of rotation (O) to the end of the recorder (a); S is the length of the recorder from the point of rotation (O) to the attachment point of the muscle and load (С).
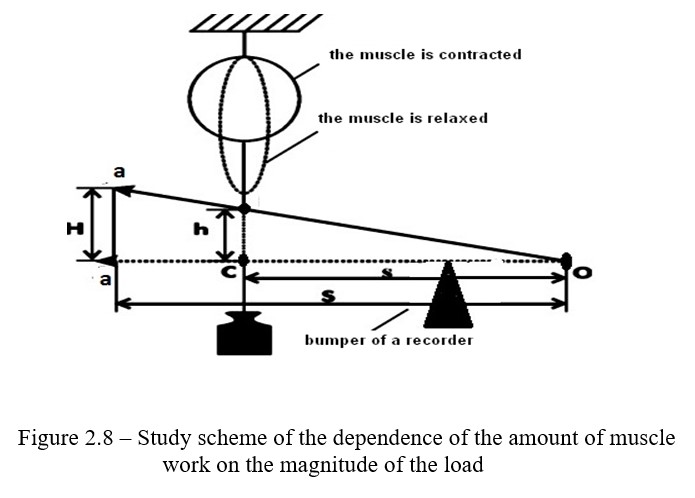
The dependence of the amount of muscle work on the magnitude of the load can be experimentally proved and expressed in the form of a graph. An apparatus for registration of muscle contractions must be assembled (Figure 2.8). For this it is necessary to use neuromuscular preparation.
The muscle must be fixed in the myograph so that it is located above the Engelman's lever. The end of the muscle with the Achilles tendon, which is below, must be connected to the Engelman's lever with a hook. A pen is attached to the front arm leverage. Various weights (10, 20, 50, 100, 200, 300, 400 g) are suspended from the same arm to the recording lever, each time applying irritation to the muscle with the help of an electrostimulator. Record the height of the muscle contraction on the drum of the stopped kymograph.
Work 4 Measurement of the work of the person's muscles. Ergometry
To measure the work of a person's muscles and developing fatigue, they use various ergometers, most often ergometers for recording movements of the middle finger. Such an ergometer is a flat tripod in which the forearm of the hands is fixed along with the brush. On the middle finger of the hand, which can be flexed freely, a hinge is put on the load suspended from the lace block to which the load is suspended. The lanyard is also fixed with the scribe who fusiruet on the drum kymograph the amplitude of flexion of the finger lifting the load. In this way, you can determine the height of lifting the load with your finger. Multiplying the weight of the cargo by the height of its rise (the sum of the amplitudes registered in the kymograph) you can determine the work of the muscle performed before the moment of refusal to work with complete fatigue.
The following is necessary for work: The work is different for different modes, which can be determined with the help of ergometry. For work you need: an ergograph, a kymograph, a metronome, loads of 1kg, 2kg. Etc.
Work progress: Place the test subject's hand on the ergograph board with the palm upward, so that the load can only be raised by bending the middle finger, without the involvement of other flexors. Select two loads and starting with a smaller one, ask the subject to raise his middle finger at a frequency of 30 times per minute before muscle fatigue begins. After rest, do the same procedure with a large load.Then do these operations with the same loads, but with a frequency of 60 times per minute. Count the number of cuts in each case and calculate the work done at a different load and frequency.
Make a conclusion about the dependence of external mechanical work performed by the human muscle on the magnitude of the load and the rate of motion.
Control questions
- Classification of muscles. Physiological features of muscles?
- The mechanism of muscle contraction?
- Types of muscle contraction?
- Muscle contraction regimen?
- Strength and work of muscles under static and dynamic loads?
- Muscle fatigue