10 Physiology of digestion
In the digestive system, processes are performed that ensure the processing and assimilation of nutrients, which is necessary to replenish the energy expenditure of the organism.
Secretory cells of digestive glands produce enzymes that break down nutrients: hydrolyse proteins, carbohydrates and fats to monomers (amino acids, monosaccharides and fatty acids).
Monomers are transported from the digestive canal to the internal environment of the body. The secretory and motor function of the gastrointestinal tract is controlled by the nervous and humoral systems.
10.1. Splitting of starch with saliva
Digestion is carried out with the help of enzymes - biological catalysts that make up the digestive juices. Enzymes are highly specific. In human saliva, amyloytic enzymes of amylase and maltase are contained. Amylase cleaves glycogen up to maltase, and maltase splits maltose to glucose. They act at body temperature (38-39 o) in a neutral and slightly alkaline environmentю
The purpose of the lesson: to study the conditions for the action of saliva enzymes and make sure that the digestion of carbohydrates by saliva is an enzymatic process.
Work 1. Action of enzymes on starch
The following is necessary for work: 1% solution of boiled starch, 1% solution of raw starch, 1% iodine solution, 1% hydrochloric acid solution, distilled water, water bath, alcohol lamp, desiccator with snow, test tube racks, measuring pipettes, Lugol's solution, filter paper .
Work progress: to study the action of the enzyme saliva on starch use
Table 10.1 – Effect of salivary enzymes on starch
|
The contents of the tubes |
Terms of Experience |
Results |
|
1) 1 ml of distilled water + 1 ml of starch paste 2) 1 ml of saliva + 1 ml of starch paste 3) 1 ml of saliva + 1 ml of raw starch 4) 1 ml of saliva + 3-5 drops of hydrochloric acid + 1 ml of starch paste 5) 1 ml of boiled saliva + 1 ml of starch paste 6) 1 ml of saliva + 1 ml of starch paste |
The first 5 tubes put in a thermostat with a temperature of + 380 - + 400 for 10 minutes.
Sixth put in the cold for 10 minutes. |
|
human saliva. Saliva is extracted by rinsing the oral cavity for 1-2 minutes with 20 ml of distilled water. The collected liquid is filtered. The numbered six tubes are prepared for the experiment as follows:
At the end of this time, 2-3 drops of Lugol's iodine solution are added to each tube. Intensive coloration in blue indicates the presence of starch.
Control questions
- Composition and properties of saliva.
- The mechanism of saliva secretion.
- Salivary glands and their nature.
- What digestive enzymes are contained in saliva and what nutrients they are acting on?
- Mucin, its chemical nature and the importance of mucin.
- How is the unconditioned saliva reflex produced?
- The role of the food center in coordinating the work of the gastrointestinal tract.
- How is the feedback between the food center and digestive organs carried out?
- The mechanism of conditioned reflex salivation.
10.2 Examination of gastric juice
To produce pure gastric juice in dogs, one of the following operations is performed:
- A) stomach fistula and esophagus transection;
- B) a small ventricle according to I.P. Pavlov.
The chemical transformation of food in the stomach occurs under the influence of gastric juice. Gastric juice is a secret of 3 kinds of glands of the gastric mucosa: the central, delomorphous and mucoid cells.
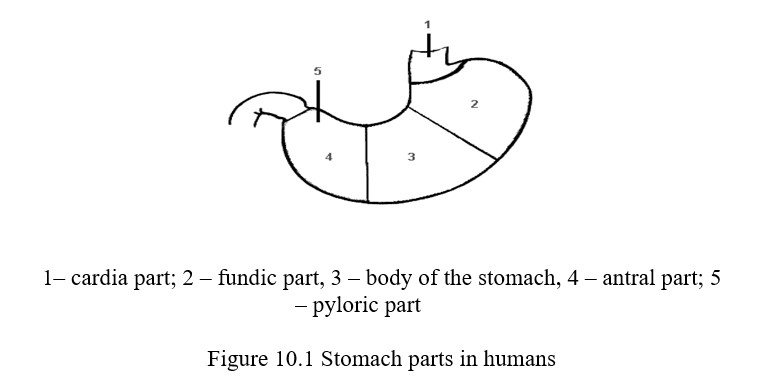
The central cells produce enzymes, the mucoid cells - mucus, the delomorphous cells - hydrochloric acid. Hydrochloric acid is in the bound state, and also in the free state.
Free and bound acid make up the total acidity.
The purpose of the lesson: to get acquainted with the forms of expression of acidity of gastric juice. Assess the physiological significance of the presence of hydrochloric acid. To get acquainted with the method of determining the digestive force by Mett's method and the methodology for determining the acidity of gastric juice. Show the presence of a pepsin enzyme in the gastric juice.
Materials and reagents: gastric juice, 0.1% solution of alkali, 10% solution of soda, litmus papers, phenolphthalein, dimethylamidoazobenzene, distilled water, fibrin, tripods with test tubes, alcohol lamps, glasses with snow, cones, pipettes, burettes, Metta' s sticks with eggwhite.
Work 1. Determination of acidity of gastric juice.
Work progress:
а) determination of free acid. 10 ml of gastric juice are poured into the flask and 2 drops of 0.5% alcohol solution of dimethylamidoazobenzene are added. In the presence of free acid, a red color is obtained, then the gastric juice is titrated with a 0.1% alkali solution until golden yellow colour. The amount of alkali in ml, which went to titration, is multiplied by 10. This will be the free acid in 100 ml. Of gastric juice.
б) determination of the combined acid. In this cup add 2 drops of 1% alcohol solution of phenolphthalein and titrate with 0.1% alkali solution until pink coloration appears. The amount of dissolved alkali in ml is multiplied by 10. This is the indicator of the bound acid. Total acidity is the amount of free and bound acid taken together.
Work 2. Conditions of action of gastric juice for protein
Work progress: number six tubes and prepare them for the experiment as follows.
Table 10.2 – The action of gastric juice on protein
|
The contents of the tubes |
Test conditions |
Results |
|
1) 3 ml of distilled water + a piece of fibrin. 2) 3 ml of 0.5% hydrochloric acid + a piece of fibrin. 3) 3 ml of boiled gastric juice + a piece of fibrin. 4) 3 ml of gastric juice + 3 drops of soda + a piece of fibrin. 5) 3 ml of gastric juice + a piece of fibrin. 6) 3 ml of gastric juice + a piece of fibrin |
5 tubes put in a thermostat with a temperature of + 38-400 for 15 minutes.
Put in the cold for 15 minutes. |
|
After this time, determine in which test tubes the protein was digested and write down the conclusions.
Work 3. Determination of the digestive power of gastric juice by the method of Metta.
Work progress: In special cups, prepare the gastric juice to be tested as follows: take 3 ml of hydrochloric acid (0.2% solution) and add 1.0 ml of gastric juice. Then drop 2 protein metta sticks of 1-1.5 cm in length. Then the cups are placed in a thermostat for 24 hours at a temperature of 37-38 0С. At the end of this period, the cups are removed from the thermostat and the juice is poured from them. Metta's sticks are laid out on a glass plate laid on black paper and put each wand to a millimeter ruler with divisions. Count the millimeters of the digested protein on the ruler from both ends of the wand and the digestive juice being examined.
Control questions
- What is the difference between an isolated ventricle according to Pavlov from an isolated ventricle according to Gaidenhain?
- Phases of gastric secretion: a) reflex; b) humoral-chemical; с) mechanical; d) intestinal;
- The course of the unconditional and conditional reflex arc secretion of juice in the stomach
- What is the essence of the "imaginary" feeding method and for what purpose did Pavlov apply this method for the first time?
- By what nerves are the efferent effects on the gastric glands, what is their effect?
- Where are the centers of the secretory nerves of the stomach?
- Importance of hydrochloric acid in the transition of the contents of the stomach into the intestine.
10.3 Absorption of nutrients from the intestine
Absorption is the physiological process of the penetration of various substances into the blood or lymph through biological membranes. In the digestive canal, food degradation products in the form of aqueous solutions are absorbed into the blood and lymph through the epithelial cells of the small intestinal mucosa (Figure 10.2).
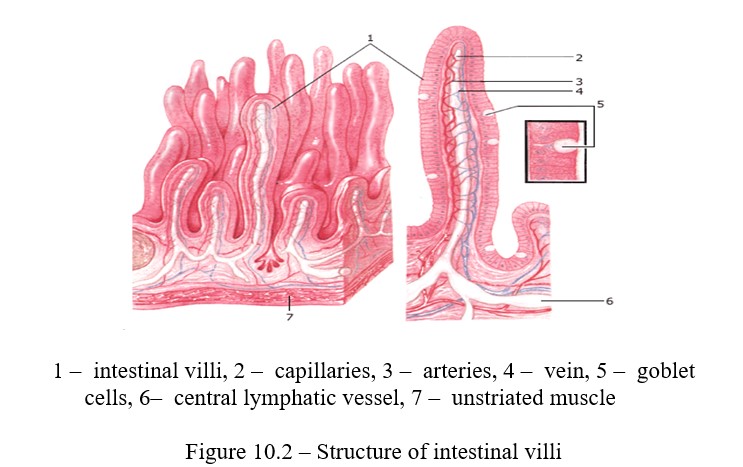
The rate of absorption of nutrients depends on their nature, osmotic pressure and the properties of the living membrane. Biological membranes have selective and one-sided permeability.
The purpose of the lesson: to study the mechanism and rate of absorption of various nutrients in the intestine and make sure that the biological membranes have a one-sided permeability.
Work 1 One-sided permeability of frog skin
During absorption, one-sided penetration of a number of substances through the epithelium of intestinal villi occurs. Similar phenomena can be observed in other organs, for example, in some sections of the renal tubules, in the frog skin. The shell of each cell has one-sided, and moreover, selective permeability. All these phenomena, including absorption through the intestinal wall, occur with the obligatory participation of intracellular exchange processes.If these processes are disrupted in the intestinal mucosa (for example, to cool the tissue to 0 ° or to poison it with sodium fluoride), then the previously observed selectivity and homogeneity of the penetration of substances through the non-living semipermeable membrane disappear. This work reveals the one-sided passage of some substances through living epithelial tissue.
The following is necessary for work: 4 cups, 4 glass tubes, 2 tripods with clamps, saline solution, 1% methylene blue solution, a set of tools, alcohol, frogs.
Work progress: destroy spinal cord of two frogs and the skin the paws and prepare 2 pouches, two of them are turned out. One inverted and one normal pouches are placed in alcohol for 2-3 minutes. Then each pouch is fixed in a tripod. All pouches from the frog's paws are filled with a 1% solution of methylene blue and dipped into cups with saline solution. Establish in which direction the output of methylene blue is carried out into the solution.
Control questions
- The essence of parietal digestion
- Phases of pancreatic juice
- What is the importance of bile in digestion
- Composition of pancreatic juice
- General principles and mechanisms for the regulation of digestion
- Absorption mechanisms in the gastrointestinal tract
- Is the absorption process regulated by the nervous system?
- How is the coordinated work of all departments of the digestive system carried out?